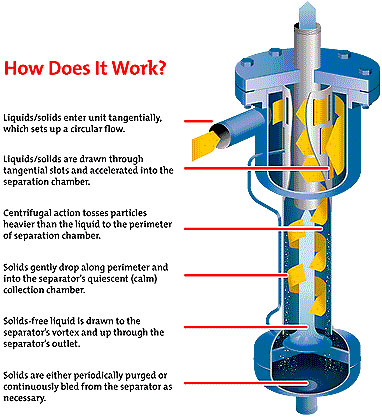
The traditional method of
treating potable stored water to protect against the infestation of
Legionellosis is through the use of chemicals.
Many companies are now looking
for an alternative to chemical dosing. They want to avoid the expensive
disruption to premises, toxic presence in the water, hazards in handling
and use, and the inevitable damage to the environment that stem from
their use.
Safewater Services Ltd.
decided to investigate the potential for alternative treatment media,
which provide the required protection without the downside. Our ongoing
development and research has identified a variety of media that can
be adapted to provide protection in a range of different situations.
For the specific treatment
of potable stored water we decided to focus on two particular media
types, each able to perform the function quite adequately. However,
when used together, the complimentary effect gives significant benefits.
It is this combination of media which provides the 'active ingredient'
for the HydroMaster™ 2000 modules.
The media used are 'Special
Active Ceramics' and a 'Noble Metal Matrix'.
The following pages provide
information pertaining to each specific media and how they work, together
with some background information and Log Kill data. The media have
undergone evaluation at the WRc Evaluation and Testing Centre (NAMAS
accredited Laboratory) and have passed 'full tests of effect on water
quality'. Subsequently, they have been classified as an approved product
under the Water Byelaws Scheme and are 'suitable for use in contact
with potable water'.
Firstly, you can find some
brief information regarding Legionella Pneumophila.
Legionella Pneumophila
What does Legionella look
like?
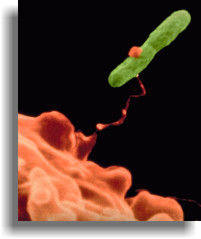 Legionella
Pneumophila is a Gram-negative bacterium that is widely distributed
in natural and manmade freshwater habitats. All members of the genus
have small, rod-shaped cells 1-2 µm in length and 0.5 ?µm
wide and require iron and cysteine for isolation. They will initiate
growth on artificial media only over a narrow pH range of 6.8-7.0
but can tolerate a pH range from 5.5-9.0 in natural habitats.
Legionella
Pneumophila is a Gram-negative bacterium that is widely distributed
in natural and manmade freshwater habitats. All members of the genus
have small, rod-shaped cells 1-2 µm in length and 0.5 ?µm
wide and require iron and cysteine for isolation. They will initiate
growth on artificial media only over a narrow pH range of 6.8-7.0
but can tolerate a pH range from 5.5-9.0 in natural habitats.
When Legionella Pneumophila was first isolated, it was found to be
only distantly related to other bacteria and was placed in its own
family, the Legionellaceae. DNA-DNA hybridization experiments and
16S rRNA studies have shown that the species of Legionella are closely
related to one another but distantly related to other bacteria. The
most closely related groups are the purple sulphur bacteria, the Enterobacteriaceae
and Pseudomonas.
Legionella Pneumophila was
identified in 1979 following an outbreak of 'Legionnaire's Disease'
caused by the bacterium. It was later learned that previous outbreaks
of Legionnaire's disease had occurred as early as 1957. Legionella
Pneumophila was isolated in 1947 in a guinea pig that had been inoculated
with blood from a patient with an unknown disease.
Legionnaires Disease
The first identified outbreak
of Legionnaires Disease occurred during a Pennsylvania State Convention
of the American Legion in 1976. 182 cases resulted in 29 deaths within
the hotel. 38 cases were reported amongst passers by, resulting in
a further 5 deaths. In recent years 200 - 300 cases of the disease
have been reported each year in England and Wales. The majority of
outbreaks are associated with buildings such as; Hotels, Factories,
Hospitals, Nursing Homes and Office Blocks.
More research has been carried
out in the United States where, according to the OSHA (Occupational
Safety and Health Administration - part of the U.S. department of
Labour), Legionnaire's disease is considered to be fairly common and
serious, and the Legionella organism is one of the top three causes
of sporadic, community-acquired pneumonia.
Because it is difficult
to distinguish this disease from other forms of pneumonia, many cases
go unreported. Approximately 1,000 cases are reported annually to
the CDC (Centre for Disease Control and Prevention), but it is estimated
that over 25,000 cases of the illness occur each year and cause more
than 4,000 deaths.
This is in excess of 25
times the number of reported cases, which would indicate that some
5000 - 7500 cases in England and Wales is, perhaps, a more accurate
figure. There are sources in the UK that believe the true problem
attributable to all of the 20+ different varieties of Legionella linked
with human diseases, could be significantly higher than this.
In the UK 180,000 people
die from all of the different varieties of pneumonia each year. As
many cases apply to people in susceptible groups (such as the elderly,
smokers, alcoholics, cancer sufferers and other immuno-suppressed
patients) rarely is a full investigation of the true cause of the
pneumonia carried out.
How do people contract Legionella?
The most popular theory is that the organism is aerosolised in water
and people inhale the droplets containing Legionella. However, new
evidence suggests that there is another way of contracting Legionella.
It appears that "aspiration" may be the way the bacterium
enters into the lungs. Aspiration means choking such that secretions
in the mouth get past the choking reflexes and instead of going into
the oesophagus and stomach, mistakenly, enter the lung.
Special Active Ceramics
What is an 'active' ceramic.
Most basic ceramics such
as glass, porcelain, clay ware, and brick, are based on natural aluminium
silicates, which are 'inactive' electrical and thermal insulators.
New technology has led to a range of 'special' or 'active' ceramics
which display physical properties of semi-conductivity, thermal and
ultra-sound conductivity, magnetic properties, and light emission,
achieved by the addition of various selected transition elements and
sintering at very high temperatures.
These 'active' ceramics,
used for the treatment of water and other liquids, are produced as
spheres, having a layered structure around a central nucleus or 'seed'
and a complex open structure, which can exchange ions (zeolyte), through
minute electrolytic cells, which become active when in contact with
an electrolyte such as water.
The material of the spheres
is approved by the Water Research Centre under the Water By-laws scheme
for use in contact with potable water.
They present no hazard to
health or body in either their use, handling, storage or transportation
(COSHH Regulations and Occupational Exposure Limits).
How Do They Work?
Most bacteria have a short
life expectancy and, deprived of nutrition or the wrong environmental
conditions, quickly expire. They reproduce by one of two methods;
binary fission, where individual cells continually divide into two
identical cells, and sexual, where two cells merge before producing
progeny by division or 'budding'. The function of any bacteriastat
is to prevent or inhibit both types of reproduction.
Disinfection can be achieved
by physical or chemical means, involving destruction of the information
required by the cell to survive (the DNA complex), or of the membrane
enveloping the cell. Physical methods include heat (wet or dry), electro-magnetic
radiation (infra-red, ultra-violet, ? & ? rays), ultra-sound etc.
These can kill all living microorganisms, the result being termed
`Sterilisation'. Chemical methods include strong oxidising agents
such as chlorine and its oxides, bromine, iodine, hydrogen peroxide
and its derivatives. Also, heavy metal ions such as those of copper,
silver, mercury etc. and specific organic compounds such as pesticides,
phenolic compounds, organo-chlorine and phosphorus. These tend to
act in a general fashion by attacking the cell as a whole, or selectively
by altering the genetic structure, and can vary in strength from mild
to strong. These are termed `Disinfectants'.
Active ceramics are a recent
development in the production of specially designed, dedicated ceramics.
When immersed in water these 'Active Ceramics' display physical properties
of semi conductivity, magnetic properties and light emission (in the
far infra red spectrum).
The effect is similar to
a miniature electric cell, the current flow causing hydroxyl ions
(OH) to convert to oxygen gas (O2). At the same time the formation
of hydroxyl and anolyte result in a neutral Ph.
The radiation emitted by
the spheres in the far infra red region of the electro magnetic spectrum
is too low to cause sterilisation, but is sufficient to excite the
molecules in the water, thereby stimulating the oxidisation process.
The oxidisation (and production
of chlorine) by the electrolysis of water acts as a potent disinfectant,
with the anaerobic bacteria (amongst which are Legionella and E.Coli)
being the most sensitive to attack and therefore most quickly controlled
or eliminated. Aerobic bacteria can also be affected but the protective
enzymes they produce considerably extend the time taken to achieve
the same result.
Bacteria are attracted by
the chemical activity and extensive substrate of the Active ceramics
where they attach themselves to the surface. There the products of
the electrolytic process destroy the D.N.A. and/or the membrane enveloping
the cell, whereupon their ability to thrive and reproduce is ended.
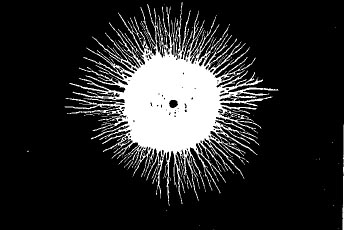
Far Infrared Emissions
from a Single Special Active Ceramic Sphere
(Autoradiograph on a photographic emulsion)
The Electro-Chemical Action of 'Active' Ceramic Spheres
Application of an electrical
voltage of a certain value across two inert electrodes immersed in
water will cause current to flow involving ions (not electrons as
in metals and semiconductors), the positive hydrogen ions (H+) collecting
at the Cathode (-ve) where electrons convert the ions to hydrogen
gas (H2). This is only possible if a corresponding process takes place
at the Anode, which transfers electrons from the water, achieved by
the Hydroxyl ions (OH) converting to oxygen gas (O2). Impurities in
the water increase the electrical conductivity of the water and reduce
the potential at the electrodes.
The region around the cathode
is called the catholyte and is generally alkaline (high pH) due to
the formation of the hydroxyl, while the region around the anode is
the anolyte, which is acidic (low pH) and is where the oxidising entities
are formed. When catholyte and anolyte mix the result is pH which
is neutral, while some of the active species lose their potency.
Using natural water with
a degree of mineralization, for example, NA+, K+, Ca+, Mg2+, C1-,
SO42-, HCO32-, etc..
ANOLYTE
pH = 3 '7 ORP = +700 '+1200
mV
Active products synthesised:
HO2, HO*2, O3, O*2, H2O2, O2, H+ (H3O+), O*, OH*, Cl2O, C1O2,
HC1O, C1O*, C1* C12, S2O82-, C2O62-
.
CATHOLYTE
pH = 10 '11 ORP = - 500
' - 800 mV
Active products synthesised:
NaOH, KOH, Ca(OH)2, Mg(OH)2, HO*, H3O2*, HO*2, H2O*2, O*2, HO-, O22-,
O2-.
Note: * = free radical
Properties
The Active Ceramics transmit
radiation, in the far-infra red region of the electro-magnetic spectrum,
of low intensity and energy (of relatively low energy compared, say,
to that of ultra-violet), too low to cause sterilisation but capable
of exciting the molecules of water by vibration and rotation, and
so increasing their mobility (by lowering viscosity) and thereby facilitating
the oxidising process.
However, it is the electro-chemical
property, which achieves disinfection. Over the surface of the ceramic
minute cells are formed, comprising pairs of cathodes and anodes,
where water is electrolysed, splitting into its component hydrogen
and oxygen albeit in a complex manner.
While the hydrogen readily
escapes, the oxygen so produced provides a powerful oxidising reagent
capable of inhibiting the growth of micro-organisms and, indeed, killing
them. This is akin not only to the physical sterilisation performed
by heat, ultra-violet, ? and ? radiation and ultra-sound, but also
the disinfecting properties of such powerful oxidants as chlorine,
bromine, iodine, chlorine dioxide, peroxide and ozone, without leaving
the latter group's obnoxious and often hazardous residues.
The significant advantage
of Active Ceramics over other (non sterilising) products currently
available for prevention of the infestation of bacteria is that the
electrolytic process (Bactericidal action) begins immediately upon
immersion, and is continuous and consistent thereafter, whatever the
operating conditions. This compares with the effects of the most commonly
used chemical treatments, which are transient and uncertain, requiring
constant monitoring and re-dosing to ensure protection.
As the pH of the water treated
is changed towards neutral the deposit of scale is
immediately inhibited, and removed over time, down stream of the influence
of the
Active Ceramics. This is shown by the formation of fine deposits in
waters of measurable 'hardness' (lime scale).
Disinfecting Capability
Most common disinfectants,
such as sodium hypochlorite, take a significant time to kill the total
number of microorganisms present, depending on their size and structure.
For example, a 1% (10,000 mg/litre) solution of free chlorine can
take up to 10 minutes to achieve 100% eradication, while the normal
1mg/litre in municipal water supplies can take as much as 24 hours
to give total kill, by which time it has been considerably diminished
by side reactions.
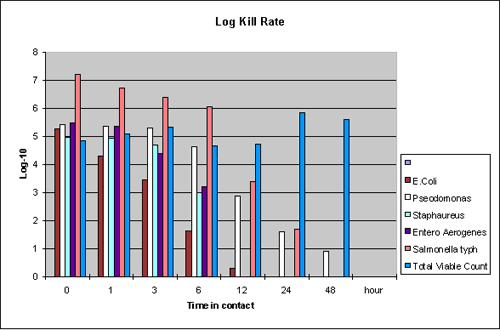
In comparison, the effectiveness
of one of the 'Active Ceramic' formulations is demonstrated in the
following graph:
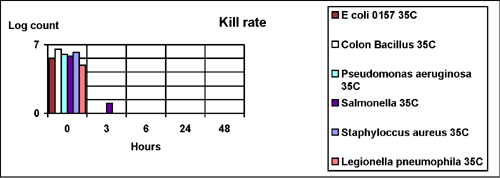
Note: Other formulations
achieve 100% kill in 24 hours but at different rates and microorganism
selectivity.
The Noble Metal Matrix
Introduction
A Noble metal (such as the
silver used in our matrix) is chemically inert or inactive, especially
toward oxygen. It has superior properties (e.g. highly resistant to
corrosion) and is usually of a relatively higher value than so called
base metals (such as iron), which tend to be of comparatively low
value and have inferior properties (such as lack of resistance to
corrosion).
In a world concerned with
the spreading of virus and disease, silver is increasingly being tapped
for its bactericidal properties and used in treatments for conditions
ranging from severe burns to Legionnaires Disease.
While silver's importance
as a bactericide has been documented only since the late 1800s, its
use in purification has been known throughout the ages. Early records
indicate that the Phoenicians, for example, used silver vessels to
keep water, wine and vinegar pure during their long voyages. In America,
pioneers moving west put silver and copper coins in their water barrels
to keep it clean.
In fact, "born with a silver spoon in his mouth" is not
a reference to wealth, but to health. In the early 18th century, babies
who were fed with silver spoons were healthier than those fed with
spoons made from other metals, and silver pacifiers found wide use
in America because of their beneficial health effects.
Recent research compared
silver-copper ionisation with the use of high temperatures to destroy
bacteria. Contaminated cold water re-infected the hot water system
even when temperatures in hot water heaters reached as high as 60
degrees Centigrade. But experiments showed that even at lower water
temperatures, ionisation of soft water with silver and copper ions
was effective against the bacteria.
'Ionisation showed better results' said Nigel Pavey, principal research
engineer for BSRIA Water Services Technology Centre in Berkshire and,
to make certain its benefits are widespread, 'there should be more
emphasis on copper-silver ionisation in legislation'.
How It Works
Our unique Noble Metal Matrix
(Patent Pending) is comprised of numerous separate woven strands of
wire manufactured from pure silver and copper. These strands are then
compressed into a matrix format that has a considerable surface area,
yet does not restrict the flow of water into which it is immersed.
Once in contact with water the differences in electromotive series
between the metals cause the release of minute quantities of the metal
ions allowing for their uptake by any microorganisms.
The common methods of treatment
(adding dilute solution or releasing the metals electrochemically)
are fraught with problems, particularly in controlling the final concentrations
in the treated water, as both copper and silver are toxic to humans
and other vertebrates. Controlling the final concentrations using
electrochemical ionisation in areas of hard water is particularly
difficult due to electrode scaling.
The Noble Metal Matrix offers
an improved disinfection method, overcoming many of these problems,
by releasing a strictly controlled quantity of ions into the water.
For over two millennia it
has been known that, copper and silver purified water. It was found
that storage of water in copper vessels prevented the growth of algae,
while silver kept the water potable. However, it was only a hundred
years ago that the oligodynamic action of such metals on biological
processes became understood.
It is now known that silver
attaches to sulphur atoms which link the helices of DNA in individual
cells of bacteria thus preventing replication and growth, while copper
has a pronounced effect on the photochemical reproduction of algae
and certain bacteria. It is known that there is synergism between
the two metals, as with other chemical species, which further oxidise
and complex the metals' ions rendering them more soluble in water.
Disinfecting Capability
The effectiveness of silver
as a bactericide is well known and researched. Tests carried out using
our Noble Metal Matrix showed the following results. At all times
the levels of silver and copper in the water remained well within
The Drinking Water Directives.
The Benefits of combining
the two media
Both the 'Special Active Ceramics' and the 'Noble Metal Matrix' are
independently capable of providing protection against bacterial infestation
and, subsequently, satisfying a 'Duty of Care'.
However, there are limitations
in the use of each individually. For instance, each individual media
demonstrates variations in the kill rate of the different bacteria.
The 'Special Active Ceramics' treat the stored water tanks but have
little residual downstream effect. They will stop the formation of
bio-film but not that of algae. The 'Noble Metal Matrix' doesn't balance
the pH of the water nor will it prevent the formation of biofilm.
 Legionella
Pneumophila is a Gram-negative bacterium that is widely distributed
in natural and manmade freshwater habitats. All members of the genus
have small, rod-shaped cells 1-2 µm in length and 0.5 ?µm
wide and require iron and cysteine for isolation. They will initiate
growth on artificial media only over a narrow pH range of 6.8-7.0
but can tolerate a pH range from 5.5-9.0 in natural habitats.
Legionella
Pneumophila is a Gram-negative bacterium that is widely distributed
in natural and manmade freshwater habitats. All members of the genus
have small, rod-shaped cells 1-2 µm in length and 0.5 ?µm
wide and require iron and cysteine for isolation. They will initiate
growth on artificial media only over a narrow pH range of 6.8-7.0
but can tolerate a pH range from 5.5-9.0 in natural habitats.